Matter - Mixtures & solutions
Last edited - July 18, 2025
A unit with lesson plans, activities, & lab notes for the middle grades
Questioning is the beginning of all learning.
- Introduction
- Big ideas, concepts, facts, & outcomes
- Science content concepts & outcomes
- Inquiry, process, & cross cutting concepts & skills & outcomes
- Pedagogical information
- Unit activities sequence
- Focus questions
- Materials
- Scoring guide suggestions
- Lesson plans
- Activity 1 - Paper clips & coins
- Activity 2 - Salt & sugar mixture
- Activity 3 - Make a mixture or separate a mixture
- Activity 4 - Properties of solutions
- Activity 5 - Mixing liquids
- Activity 6 - Sugar & water
- Activity 7 - Sand & salt water
- Activity 8 - Dissolving solids in warm & cold water
- Activity 9 - Skittle chromatography
- Lab notes
- Lab note 1 - Paper clips & coins
- Lab note 2 - Salt & sugar mixture
- Lab note 3 - Make a mixture or separate a mixture
- Lab note 4 - Properties of solutions
- Lab note 5 - Mixing liquids
- Lab note 6 - Sugar & water
- Lab note 7 - Sand & salt water
- Lab note 8 - Dissolving solids in warm & cold water
- Lab note 9 - Skittle chromatography
- Properties of Mixtures & Solutions fact sheet
Introduction
This plan investigates mixtures and solutions and their properties as states of matter. Properties of how they mix and do not mix, take up space, have volume, mass, exert pressure, flow through space, and their attraction to itself and to other particles.
It is one unit from of a sequence of units that investigate matter to review and develop a deeper understanding of its properties and states: solid, liquid, and gas.
It includes detailed plans, suggestions, solutions, worksheets, or lab notes, and material list of items which are easy to obtain from home or thrift stores. While the scope of the sequence of activities is very comprehensive, for a middle level unit, the activities may be used in many other ways or combinations for more targeted learning experiences.
Related activities
- Air & water - as Matter - solid liquid, & air/gas
- See Inverted Jar With Two Holes Investigation which includes an extensive list of learner responses for different levels of understanding for how water does and does not enter an inverted jar.
- Bubbles
- Liquid - water
- Water themed unit
- Pressure
- Heat energy activities
- Heat energy hot cold - Learning cycle inquiry lesson with lesson plans, examples, video, materials, and lab notes to explore: hot & cold with touch, a variety of activities using water, mixing hot & cold water, warming ice water to boiling, salt & ice, temperatures of cars in sunshine, blowing hot & cold bubbles, conduction, convection, & radiation.
- Heat energy and conservation - candle and heat
- Plasma
Planning information
Learner background information
A plan designed for learners who have prior knowledge in cause and effect. The use of observations to make inferences, models as explanations for observable and non observable events, change, relative position, and working in groups is helpful, but not necessary. Basic understanding of solid, liquid, and gas as in (matter unit) is helpful.
Intended learnings & learner's thinkings
Content Big ideas, concept & facts, & outcomes
(Source concepts & misconceptions)
Big ideas and specific outcomes:
We understand our world and make decisions based on our understanding of the physical matter in it, the properties they have and how we interact with matter, and how we can use it to make our lives better.
Concepts and facts
- Matter - solids, liquids, gases
- Mixtures & solutions
- Properties
- Equilibrium
- When objects exert equal forces on each other they are at equilibrium.
- When objects are not in equilibrium one will displace the other.
Outcome
Explain properties of water and how they are important for life on Earth.
Science concepts: physical, earth, life
Big ideas: Mixtures and Solutions have many properties:
A mixture of substances can be separated back to the original substances by using one or more of the characteristic properties (filtration, evaporation, etc.).
Solutions are a mixture where a solid dissolves in a liquid. The substance that dissolves is called a solute. The liquid in which it dissolves is called a solvent.
Compounds are different than mixtures in that they combine to form new substances. (For example salt and sugar together is a mixture because salt and sugar do not bond together. Salt itself is a compound with sodium and chloride atoms bonded together. Salt is quite different from either of these two substances.)
Related concepts & facts for liquids
- A mixture is a substance made by mixing other substances together and can be separated into the substances from which they are made. Mixtures and their substances can be combinations of solids, liquids and gases. Like carbonation in drinks, air between spaces of solid and liquid mixtures. The particles are generally larger than those in a solution. Substances can be separated using differences between one or more of the their properties (sifted, screened, filtration, evaporation, magnetism, electrical charge, ...).
- A solution is a liquid mixture where solid particles are said to dissolve in a liquid and become uniformly distributes. The substance that dissolves is called a solute. The liquid in which it dissolves is called a solvent. The particles are generally smaller than those in a mixture. Solutions can have particles (solute) of various sizes. However, solution is a term generally used when the particles of the solute can be seen with your naked eyes. Thus, there are different names for solutions with different sizes, and people may use these terms interchangeably (solution, suspension, colloid).
- Solutions are matter that take up space and have mass. It exerts a force and flows when not at equilibrium.
- The different substances in a solution can mix really well or separate when not being shaken.
- Some will stay mixed and others will separate at different rates.
- Solutions flow and spread out.
- Different kinds of solutions, depending on how thick they are, flow (spread out) at different rates,
- Solutions will flow until they interact with another object.
- When they interact with another object they can change their direction or be contained.
- Usually solutions are contained in a solid container.
- Solutions which are contained will take the shape of the container.
- When solutions are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- Solutions can be: transparent, translucent, viscous, opaque, foamy, and bubbly.
- Dissolve means to get smaller and become part of a solution. It is only a physical change of getting smaller, as opposed to a chemical change of becoming another substance. Particles can vary in size from being visible to microscopic to being single atoms or molecules.
- Solvent is a liquid that can dissolve a substance (the solute) to make a solution.
- Solute is a substance that a liquid can dissolve to make a solution.
- Mixtures and solutions are made of matter that takes up space and have mass. It exerts a force and flows when not at equilibrium.
- Solutions flow and take the shape of a container. And if they are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- Chromatography is the separation of a mixture by passing it in solution (or suspension or gas) through a medium in which the components flow at different rates.
- Different concentrations may have different or similar densities.
- Concentration is the amount of a given substance within a solution.
- A suspension is a solution of two or more substances where the solution is opaque to light, the particles are visible to the naked eye, and settle down under the influence of gravity. A suspension has particles generally smaller than those referred to as a solution and can be separated out by ordinary filtering or centrifuging.
- Colloids are suspensions of two or more substances where the solute particles remain suspended throughout the solution (bouillon cube, milk, paint, blood, fog, whipped cream, perfume, gels) and is often transparent or translucent to light. Some colloids can be classified on the basis of the state of matter (emulsion, foam, aerosol) of the dispersed substance and the state of matter in which it is dispersed. The colloidal suspension with solid particles in a liquid is known as colloidal solution. the particles do not settle, are mostly microscopic, and cannot filtered easily.
- An emulsion is a mixture of two liquids.
- Foam is formed when many gas particles are trapped in a liquid or solid.
- Aerosol contains small particles of liquid or solid dispersed in a gas.
Related concepts for solids, liquids, and gases.
- Solids, liquids, and gases are a state of matter (solid, liquid, gas, & plasma). Therefore, it takes up space (volume) and has mass.
- Solids, liquids and gases exert a force or pressure.
- Liquids and gases flow when they exert unequal pressure on each other.
- When pressures are equal they are at equilibrium.
- Solids, liquids, and gases exert more pressure at the bottom than at the top.
- Liquids pour, flow, and spread out.
- Liquids will flow to the lowest possible space.
- Liquids will flow until they interact with another object.
- Different kinds of liquids, depending on how thick they are, flow (spread out) at different rates.
- When they interact with another object they can change their direction, move other objects (matter), solids, liquids and gases, or be contained. Because no two objects can occupy the same space at the same time.
- No two objects can occupy the same space at the same time.
- Liquids can be pushed out of the way by solid objects, other liquids and gases.
- Usually liquids are contained in a solid container.
- Liquids which are contained will take the shape of the container.
- The surface of the liquid is level to the ground.
- Different liquids can have different and similar densities.
- When liquids are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- The properties of liquids are transparent, colored, opaque, viscous, translucent, bubbly, and foamy.
- Liquids have a temperature that is a measure of heat energy.
- Liquids of different temperatures have different densities.
- When water particles get near each other they attract which gives them some interesting qualities such as adhesion, cohesion, capillary action, and surface tension.
- The attraction and attaching of water to itself is called cohesion.
- The attraction and attaching of water to something else is called adhesion.
- The strong attraction of water particles at the surface, to itself and other objects is known as surface tension.
- The attraction of water helps particles move upward in tubes or spaces, which is known as capillary action.
Outcomes
- Use accurate verifiable information to consider properties of mixtures and solutions and how understanding them can influence us in making decisions.
- Describe mixture as a substance made by mixing other substances together that can be separated into the substances that it is made of.
- Describe solution as a liquid mixture in which the minor component (the solute) is uniformly distributed within the major component (the solvent).
- Describe mixtures and solutions as matter that takes up space and have mass. It exerts a force and flows when not at equilibrium.
- Describe that solutions flow and take the shape of a container. And if they are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
Anticipated learner thinkings & misconceptions
Misconceptions for matter
Scoring guides suggestions (rubric)
Mixtures & solutions as matter (scoring guide)
Top level
- Describe mixtures and solutions as combinations of matter (By knowing it takes up space, has mass, and will flow downward unless there is matter to stop its flow). It exerts a force and flows when not at equilibrium. When it flows it can be stopped by a solid, a more dense liquid or a gas under pressure). When mixed together, they can be separated. Explain, with a model that shows how properties: opaque, transparency, translucent, flow ... of solutions or mixtures change according to the difference in the size of the components that make up the solute and the viscosity of the solution.
- Describe mixtures and solutions as matter that is mixed together and takes up space and has mass.
- Describe mixtures and solutions as fitting the shape of a container. And if they are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- Mixtures and solutions are made of different substances blended / together that take the shape of a container.
Lower level
Inquiry, process, & cross cutting concepts & skills
Inquiry (How science inquires for understanding: process, skill, methodology, practice)
When I experiment I collect observations of properties to describe different objects and can use those properties to describe change (when properties change they become variables) when objects and systems interact. This helps me make claims to explain what is happening, and make models to predict what might happen in the future.
Related concepts and facts
Properties of objects are determined by the elements from which they are made. Properties can remain constant, change, and be measured. They are used to identify objects, as variables in experiments, operational definitions, and explanations. Properties of matter include: color, texture, size, shape, mass, volume, density, temperature, chemical, energy, states of matter (solid, liquid, gas, plasma) and the ability to interact with other objects. Properties can be measured with scientific tools and compared to a standard unit (linear, time, temperature, mass, volume, and density)
- Observed changes can be described as changes of properties (variables).
- Variables are observations of a property that changes - size, shape, temperature, amount, volume, rate, ...
- When people disagree on an observation, they usually make more and better observations.
- Observation, creativity, and logical argument are used to explain how variable changes effect resulting observations.
- Observed changes can be explained as being caused by changes of variables (changes of properties/ characteristics).
- Explanations are based on observations.
- Evidence is observation.
- Inference is an explanation based on observation.
- When people disagree on explanations, they can make more observations or change their explanation.
- Better decisions are made when information is verified before being considered accurate and used to reason and develop explanations and models to understand the world and make decisions.
- People make better decision when they understand and consider the positive and negative influences that effect their decision making.
Outcome
- Use accurate verifiable information to consider properties of mixtures and solutions and how understanding them can influence us in making decisions.
- Describe change as a result of interactions. Describe those interactions as changes of a characteristic/ property (variable) that interacts with the object that changes (model).
Specific outcomes -
- Describe properties (looks, transparency, flow ... ) of mixtures and solutions and use them to explain and model different mixtures and solutions by showing how solutions or mixtures change according to the difference in the size of the component solute that make up the solution and the viscosity of the solvent.
Cross cutting
Systems, Order, & Organization; Evidence, Models, and Explanations; Constancy, Change, and Measurement; Evolution and Equilibrium; Form and Function
See more proceeds concepts & misconceptions
Related concepts and facts
- Observational data and reasoning is used to explain interactions. Evidence is something that
is observed and can be used to understand what is happening and make predictions
about future changes.
- Explanations are based on observation derived from experience or experimentation and are understandable.
- Pictures or symbols can represent objects.
- Tables, charts, and graphs can be used to organize information to help understand.
- System is a group of related objects that works together for a particular purpose.
- When parts are put together they can do things they can't do alone.
Model
- Models are structures that correspond to real objects, events, or classes of events that have explanatory and predictive power (physical objects, plans, mental constructs, mathematical equations, computer simulations...) that may or may not be observed with real objects, systems, and events.
- Model is an explanation based on observations, facts, laws, inferences, thought, and reasoning.
- Models represent systems or things used as an example to follow or imitate to provide an explanation.
- Models can be used to think about events or processes that happen very slow, fast, or on a too small or large scale to change easily or safely.
- Mathematical models can be displayed on computers and changed to see what happens.
- Models are used to represent things in all dimensions of science physical, earth, and life science.
- Models are created with similar processes, which can be an algorithm or procedure. Such as in the following diagram.
- A model, though different from the real thing, can be used to learn something about the real thing.
- Seeing how a model changes may suggest how the real thing works if the same were done to it.
- Models make predictions.
- More than one model can represent the same thing or event.
- The kind of model and its complexity depend on the purpose of using the model.
- Models are never exact representations.
- A model that is too limited or complicated may not be useful.
- A model represents entities and the relationships between them.
- There are two basic types of models, physical and conceptual. Conceptual communicate through words and drawings, or can be physical and demonstrable.
- Models help generate ideas, solve problems, make predictions, help think.
- Models can be used to represent new ideas and inventions.
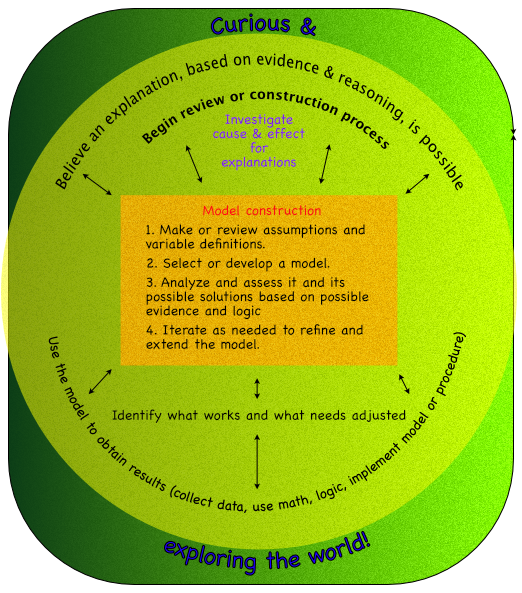
Outcome -
- Make observations of change, identify variables associated with the change, and create explanations and models of how the variables effect those changes.
Specific outcomes -
- Identify properties that change.
- Relate variables to change with an explanation.
- Generalize explanations of how properties change as models to explain future situations.
Perspectives
Engineering & Technology
- People have always had problems and invented tools and techniques (ways of doing something) for scientific inquiry and technological design.
- The two have similarities and differences.
- Scientists propose explanations for questions about the natural world, and engineers propose solutions relating to human problems, needs, and aspirations.
- Technologies exist within nature and so they cannot contravene physical or biological principles. Technological solutions and technologies they have side effects, costs, carry risks, and provide benefits.
- Many different people in different cultures have made and continue to make contributions to science and technology.
- Technology is essential to science, because it provides instruments and techniques that enable observations of objects and phenomena that are other wise unobservable due to factors such as quantity, distance, location, size, and speed.
- Technology provides tools for investigations, inquiry, and analysis.
- Science and technology are reciprocal.
- Science helps drive technology, as it addresses questions that demand more sophisticated instruments and provides ideas for better instrumentation and technique. Technological designs have constraints that engineers, architects, and others must take into account to solve practical problems.
- Some constraints are unavoidable, for example, properties of materials, or effects of weather and friction; other constraints limit choices in the design, for example, environmental protection, human safety, and aesthetics.
- The knowledge base for science is recorded in print and electronic media and can be found and understood by people in and out of classrooms
Personal and Social Science
- The application and the use of models in science and technology can and will benefit society.
- Model helps me internalizing or abstracting understanding.
Nature of Science
- Science can provide ideas for our protection and desire for a better life.
- Scientists use models.
History of science
See also Concepts & misconceptions also science, math, technology timeline
- People have practiced science and technology for a long time.
- Science develops over time.
- Science investigators such as
Strategies and activities to achieve intended learnings
Are designed based on the learning cycle theory & method
Pedagogical Overview
Supporting information
Information in this plan is written for teachers and other adult readers. For learners, concepts such as, mass, volume, density, molecules, ... require much experience with physical objects as models and the development of logical thinking. As learners develop these concepts starting with concrete objects for which they are familiar and moving toward more formal reasoning can be a good way to scaffold their understanding. Such as molecules, which might be more appropriately referred to as pieces of water, water particles, etc. The terms cohesion and adhesion could be termed as stickiness and could be used interchangeably. The correct use of terminology and full understanding in cases like these would not be expected until junior high.
Activities Sequence to provide sufficient opportunities for learners to achieve the targeted outcomes.
Make sure learners have the prior knowledge identified in the background information.
- Activity 1 - Paper clips & coins
- Activity 2 - Salt & sugar mixture
- Activity 3 - Make a mixture or separate a mixture
- Activity 4 - Properties of solutions
- Activity 5 - Mixing liquids
- Activity 6 - Sugar & water
- Activity 7 - Sand & salt water
- Activity 8 - Dissolving solids in warm & cold water
- Activity 9 - Skittle chromatography
Focus question
Unit focus question:
What is a mixture and solutions? How do we use them and respect it?
Sub focus questions:
- What is matter?
- What is a mixture?
- what is a solution?
- What are the properties of mixtures and solutions? Are two or more different kinds of matter combined that occupies space, has mass, exerts a force or pressure.
- How are mixtures and solutions made / combined?
- How are mixtures and solutions separated?
- What causes them to flow?
- What happens when liquids are mixed?
- What happens when solids are mixed?
- What happens when solids and liquids are mixed?
- What happens when different liquids of different or similar densities are mixed?
Materials
- Clear cups, clear bottles with lids, paper towels,coffee filters, plates, paper clips, magnet, coins, magnifying lenses, water - warm & cold, teaspoons, salt, sugar, sand, soil, flour, cocoa, tea, coffee, pepper, baking soda, baking powder, stir sticks
- Fruits, cereal, & vegetables, bowl of party mix, variety of drink mixes, and maybe brownie ingredients
- Liquid hand soap (white), dish soap (blue or green), oil, Italian salad dressing, cooking oil, corn syrup, food coloring
- Skittles
Resources and Materials
- Lab note 1 - Paper clips & coins
- Lab note 2 - Salt & sugar mixture
- Lab note 3 - Make a mixture or separate a mixture
- Lab note 4 - Properties of solutions
- Lab note 5 - Mixing liquids
- Lab note 6 - Sugar & water
- Lab note 7 - Sand & salt water
- Lab note 8 - Dissolving solids in warm & cold water
- Lab note 9 - Skittle chromatography
Lesson Plans
Activity 1 - Paper clips & coins
Materials
- Several nickels or pennies, paper clips, magnet. NOTE a 1943 penny is magnetic, probably a rare find.
- Lab note
Focus questions
- What is a mixture?
- How do you know and why?
- How can the be separated?
Learning outcomes
- Explain how mixtures can be separated by identifying different properties and using them to sort the components of the mixture.
Suggested procedures overview :
- Put learners into groups or have learners take turns to demonstrate the investigation.
- Explore a mixture of solids. coins and paper clips.
- Explain how mixtures can be separated by identifying different properties and using them to sort the components of the mixture.
Exploration to review / assess what they know about properties of solid, liquid, & gas
- Put learners into groups or have learners take turns to demonstrate the investigation.
- Ask. Have you ever tried to separate objects in a mixture (i.e. raisins from corn flakes, marshmallows from cereal, etc.)?
- Listen to all responses. Do not comment on accuracy.
- Ask. What would you do to sort a mixture of coins and paper clips into groups without touching them?
- Ask. How could you record the data?
- Let them try to separate the nickels from the paper clips without touching them and have them record the data.
- Have them share their data / drawings.
Invention
- Ask. How can you interpret the results?
- How were the paper clips sorted? The paper clips magnetic and attracted to the magnet.
- Why weren't the nickels? The coins are not magnetic and were not attracted.
- How can we explain this? By knowing the properties of the components we can use them to discover ways to sort the different components that make up a mixture. If we know they are different sizes, we could use a screen to separate large and small components as they do when sorting gravel, sand, and other Earth materials. Similarly they can use a filter to filter out larger components from liquids. Coffee filters, tea bags ...
- Illustrate in your lab notes how the two different components (coins & paper clips) interacted or didn't with the magnets.
Discovery
- Ask. Where could you use what you learned?
- If you select a cereal high in iron, you can separate it from the cereal mixture. It is embedded in the other substances around it, therefore, it needs to be crushed to release the iron particles, so they can be picked up with a magnet. Or you could dissolve the other parts of the cereal to release more iron.
Activity 2 - Salt & sugar mixture
Materials :
- Salt crystals, sugar crystals, cups, magnifying lenses
- Lab note
Focus questions :
- How can you identify salt and sugar?
- How can you tell. Without tasting?
Learning outcomes
Describe the properties of sugar and salt.
Suggested procedures overview :
- Put learners in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity - Explore salt and sugar and identify properties that don't change when they are mixed.
Exploration
Activity:
- Put learners into groups.
- Show a container of salt and sugar.
- Ask. How can you identify salt and sugar? Listen to all responses.
- If you desire, to take the time to explore each with a hand lens, let them do so and have them draw a diagram of each and discuss how they can visually see a difference between the two crystals. salt crystals are more squarish and cloudy, Sugar crystals are more elongated, hexagonal, and more clear.
- Ask. What will happen when they mix salt and sugar together? Listen to all responses.
- Have them mix them together so they can all see them being mixed and the final results.
- Ask. How can you tell the two substances apart? Without tasting? Listen to all responses.
- Have them observe the mixture and record data.
- Ask. If you taste it will it taste like sugar & salt or will it taste totally different? sugar and salt
- Have them taste it to see that it retains both the sweet and salty tastes.
- Have them record and share their data / drawings.
Invention
- Share their drawings and discuss what happened.
- Ask them to interpret the results. The different compounds were mixed together, (a physical change not chemical) but each has the same properties as before they were mixed. It is possible to separate them so you could put them back into the original containers they were in before they were mixed, but I don't know why you would want to take the time?
Discovery
- Ask. Where could they see the same results at home? Any mixture of cereal with fruit or nuts and of course the cereal grain and maybe more.
- Next activities: surface levels of liquids, properties of liquids
Activity 3 - Make a mixture or separate a mixture
Materials :
- Variety of fruits, cereal, & vegetables, bowl of party mix, variety of drink mixes,and maybe brownie ingredients.
- CHECK for allergies ... before selecting the ingredients used. For example if a person has a nut allergy, then nuts should not be in the mix.
- Lab note
Focus questions :
- How can you make a mixture?
- How can you separate a mixture?
Learning outcomes
Describe how to make or separate a mixture and explain why it is possible.
Suggested procedures overview :
- Put learners in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity - Explore the making a mixture and separating a mixture.
- Explain a mixture can be made and separated because the components that are used to make them do not change. Therefore, they can be put together and taken apart. A physical change.
Exploration
- Put learners into groups.
- Ask. What is a mixture?
- Ask learners how they could separate the items of a mixture.
- Give the following directions:
- Group A – make a mixture using a variety of fruits, cereal.
- Group B – separate the items in a bowl of party mix.
- Group C – Make mixtures using various types of drink mixes.
- Group D – make a mixture using brownie ingredients.
- Share and discuss the results.
Invention
- Discuss how making the mixture or taking the mixture apart didn't change the properties of the components used in the mixtures. (physical change)
- Ask. How could you use what you learned? You can make a mixture say with
Discovery
Make brownies and discuss how it was a mixture before it went into the oven and after .. maybe not so much. Depends on the mix used. Chemical change?
Activity 4 - Properties of solutions
Materials
- Water, liquid hand soap (white), dish soap (blue or green), oil, Italian salad dressing, seven clear bottles. Make three or four different solutions: oil & water, soap & water, Italian salad dressing, and juice (fruit or vegetable).
- Lab note
Focus questions :
- What are the properties of solutions?
Learning outcomes
Describe the properties of solutions.
Suggested procedures overview :
- Put learners in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity - Explore the solutions.
- Describe the properties of solutions.
- Create a list of solution properties.
Exploration
- Put learners into groups.
- Display bottles of solutions.
- What are some ways we can investigate the solutions without opening the bottles? (shake, roll, tip, etc)
- Have learners explore the solutions and record their observations for each action.
- You may need to provide a chart with the action written in each cell so that they can draw a picture and write an explanation for each action.
- Have them share their observational data with the class.
- Have them classify them by their observations and provide reasons for each.
Invention
- Introduce vocabulary: transparent, translucent, viscous, opaque, foamy, and bubbly.
- Have them label their examples and explain their reasoning.
- Ask. Could a solution be placed in more than one category?
- Add properties of solutions to the property chart.
- Think of other examples of solutions and classify them accordingly.
- Ask what are some properties of solutions? The following list is provided as a resource. Learners should be guided to make their own and edit it as they continue their studies. Professional wisdom should be used in deciding how comprehensive each learner's list should be.
Properties of mixtures & solutions
- A mixture is a substance made by mixing other substances together and can be separated into the substances from which they are made. Mixtures and their substances can be combinations of solids, liquids and gases. Like carbonation in drinks, air between spaces of solid and liquid mixtures. The particles are generally larger than those in a solution. Substances can be separated using differences between one or more of the their properties (sifted, screened, filtration, evaporation, magnetism, electrical charge, ...).
- A solution is a liquid mixture where solid particles are said to dissolve in a liquid and become uniformly distributes. The substance that dissolves is called a solute. The liquid in which it dissolves is called a solvent. The particles are generally smaller than those in a mixture. Solutions can have particles (solute) of various sizes. However, solution is a term generally used when the particles of the solute can be seen with your naked eyes. Thus, there are different names for solutions with different sizes, and people may use these terms interchangeably (solution, suspension, colloid).
- Solutions are matter that take up space and have mass. It exerts a force and flows when not at equilibrium.
- The different substances in a solution can mix really well or separate when not being shaken.
- Some will stay mixed and others will separate at different rates.
- Solutions flow and spread out.
- Different kinds of solutions, depending on how thick they are, flow (spread out) at different rates,
- Solutions will flow until they interact with another object.
- When they interact with another object they can change their direction or be contained.
- Usually solutions are contained in a solid container.
- Solutions which are contained will take the shape of the container.
- When solutions are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- Solutions can be: transparent, translucent, viscous, opaque, foamy, and bubbly.
- Dissolve means to get smaller and become part of a solution. It is only a physical change of getting smaller, as opposed to a chemical change of becoming another substance. Particles can vary in size from being visible to microscopic to being single atoms or molecules.
- Solvent is a liquid that can dissolve a substance (the solute) to make a solution.
- Solute is a substance that a liquid can dissolve to make a solution.
- Mixtures and solutions are made of matter that takes up space and have mass. It exerts a force and flows when not at equilibrium.
- Solutions flow and take the shape of a container. And if they are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- Chromatography is the separation of a mixture by passing it in solution (or suspension or gas) through a medium in which the components flow at different rates.
- Different concentrations may have different or similar densities.
- Concentration is the amount of a given substance within a solution.
- A suspension is a solution of two or more substances where the solution is opaque to light, the particles are visible to the naked eye, and settle down under the influence of gravity. A suspension has particles generally smaller than those referred to as a solution and can be separated out by ordinary filtering or centrifuging.
- Colloids are suspensions of two or more substances where the solute particles remain suspended throughout the solution (bouillon cube, milk, paint, blood, fog, whipped cream, perfume, gels) and is often transparent or translucent to light. Some colloids can be classified on the basis of the state of matter (emulsion, foam, aerosol) of the dispersed substance and the state of matter in which it is dispersed. The colloidal suspension with solid particles in a liquid is known as colloidal solution. the particles do not settle, are mostly microscopic, and cannot filtered easily.
- An emulsion is a mixture of two liquids.
- Foam is formed when many gas particles are trapped in a liquid or solid.
- Aerosol contains small particles of liquid or solid dispersed in a gas.
Discovery
Review and add to list of properties of solutions as appropriate for the learners.
Activity 5 - Mixing liquids
Materials :
- Two clear bottles with lids for each group, paper towels, cooking oil, corn syrup, colored water.
- Lab note
Focus questions :
- What happens when liquids are mixed?
Learning outcomes
Describe that when liquids are mixed they can flow or they can be supported by another liquid if it is heavier (more dense). Since they are mixed, they are solutions and can be separated (physical change).
Suggested procedures overview :
- Put learners in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity - Explore liquids in different containers and what happens when they are not in a container.
- Conclude that liquids flow and take the shape of a container. And if they are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
Exploration
- What happens when two liquids are poured into the same container?
- Discuss the possible combinations of the liquids using only two at a time.
- Predict what will happen in each combination.
- Allow them to experiment with the combinations of liquids and record their observations.
Invention
- Discuss the results.
- Have them predict what would happen if all three liquids were poured in the cups in various combinations.
- Allow them to experiment with the liquids and record observations.
- Discuss the results and explanations for the reactions.
- Why is this important to know about liquids and how we incorporate them into everyday life?
Add to Properties of ...
Discovery - Oobleck, liquid or solid?
Materials
- 1 cup cornstarch, 1 cup water, paper towels
Exploration
- Ask. Is cornstarch a solid?
- Why? Each grain has a definite shape that takes up space and has mass.
- What is water? liquid
- Why? It takes the shape of the bottle and has mass.
- Predict what will happen when the two are combined. Accept all answers. Like mud or soupy white mess.
- Have them combine the ingredients to make Oobleck.
- Let them explore it to show that it flows and will support objects set on top of it and ask if they can shape it so it will retain its shape or will always take the shape of its container?
- Ask. Is Oobleck a liquid or solid? Accept all answers AND ask...
- Them to justify and explain their reasoning.
- What other objects or systems can be organized as a combination of liquids & solids? Ex: Jell-O, cake batter, glue, et
Activity 6 - Sugar & water
Materials
- Teaspoon, shallow plate or container, sugar, water, and a small container to mix them.
- NOTE: salt will not dissolve in alcohol sugar will somewhat.
- Lab note
Focus questions:
- What happens when sugar and water are mixed?
- Can they be separated? How?
Learning outcomes:
- Explain sugar and water is a mixture, because yet can be separated, mixed, separated ...
Suggested procedures overview:
- Put learners in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity - Explore sugar and water as a mixture and how they can be mixed and separate infinitely ...
Exploration -
- Put learners in pairs.
- Ask. What will happen when sugar is added to water?
- Give the learners a plate or shallow container, sugar, and water.
- Allow them put the sugar in the water and mix them. (Maybe a teaspoon of sugar and 1/4 cup of water)
- Have them record what they observe before and after mixing.
- Tell them to record what happened.
Invention -
- Review observations.
- Have them explain what happened with respect to a mixture or other... If it is a mixture, then the water and sugar is still water and sugar. They are just mixed. A physical change.
- Say. If this is a mixture, you should be able to separate the two compounds.
- Ask. How could you problem solve and find a way to separate the mixture of sugar and water?
- Have them record a procedure to try their suggestions.
Discover
Ask. When could they use this information at home? They could use it to explain how the water in their faucet leaves hard water marks or stains in their sink of pans when they boil water.
Could explore salt and alcohol to show it will not dissolve in all liquids.
Activity 7 - Sand & salt water
Materials:
- Sand, tablespoon of salt, drinking glass, water, coffee filter, plate
- Lab note
Focus questions:
- What happens when sand and salt water are mixed?
- Is it a solution?
- Can it be separate?
Learning outcomes:
- Describe how sand, water, and salt water both a solution and mixture.
Suggested procedures overview:
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity - Explore how sand and salt in water.
- Describe how sand, water, and salt water both a solution and mixture.
Exploration
- Put learners in pairs.
- Give them a drinking glass, water, sand, and salt and ask them to use these to make a mixture.
- Listen to their suggestions and have them decide on a procedure.
- When they have created a mixture, ask them to problem solve how they could separate each part of the mixture.
- List their ideas on the board.
- Let the them explore using different ideas listed on the board.
- Have them draw (before, during, and after) pictures in their journals.
- After they have separated the sand, have them share the methods they used and whether these methods were very efficient.
- Have them predict what will be left after the water evaporates.
- Have them compare predictions to actual results.
Invention
- Review and discuss the before, during, and after data.
- Have them explain how the mixture was made and how it changed after being filtered, and evaporated. Draw a model for each (before, during, after) showing the components and what happened to each of the components in the process they created.
Discover
Ask. Where do you think separating a mixture is useful? Many times water is a mixture before it can be used as drinking water. Filters and reverse osmosis is used to change it to a safe mixture with mostly water, depending on the process used.
Activity 8 - Dissolving solids in warm & cold water
Materials
- Clear cups (3-4 for each group), warm water, cold water, small amounts of soil, flour, cocoa, tea, coffee, pepper, baking soda, and baking powder, stirs
- Lab note
Focus questions
- What is a mixture?
- How do you know and why?
- What is a solution?
- How do you know and why?
Learning outcomes
- Explain and model different mixtures and solutions by describing their properties (looks, transparency, flow ... ) and how they interact according to the difference in the size of the component solute that make up the solution and the viscosity of the solvent.
- Explain that when substances mix as a physical change, they form a mixture or solution. And there are different types of solutions that vary according to the size of the particles in the mixture (solute) and the viscosity of the solvent. Size can ranging from where the particles are visible in the solution to where the particles are not visible, like salt water where the particles in the mixture are at the atomic or molecular scale.
Noted difference include:
Dissolve means to get smaller and become part of a solution. It is only a physical change of getting smaller, as opposed to a chemical change of becoming another substance. particles can vary in size from being visible to microscopic to being single atoms or molecules.
A mixture is when particles of one substance are distributed evenly throughout another substance. The particles are generally larger than those in a solution. Substances can be separated back to the original substances by using one or more of the characteristic properties (sifted, screened, filtration, evaporation, ...). Mixtures are referred to both combinations of solids, liquids, and gases.
A solution is a mixture where solid particles are said to dissolve in a liquid. The substance that dissolves is called a solute. The liquid in which it dissolves is called a solvent. The particles are generally smaller than those in a mixture. Solutions can have particles (solute) of various sizes. However, solution is a term generally used when the particles of the solute can be seen with your naked eyes. Thus, there are different names for solutions with different sizes, and people may use these terms interchangeably.
A suspension is a solution of two or more substances where the solution is opaque to light, the particles are visible to the naked eye, and settle down under the influence of gravity. A suspension has particles generally smaller than those referred to as a solution and can be separated out by ordinary filtering or centrifuging.
Colloids are suspensions of two or more substances where the solute particles remain suspended throughout the solution (bouillon cube, milk, paint, blood, fog, whipped cream, perfume, gels) and is often transparent or translucent to light. Some colloids can be classified on the basis of the state of matter (emulsion, foam, aerosol) of the dispersed substance and the state of matter in which it is dispersed. The colloidal suspension with solid particles in a liquid is known as colloidal solution. the particles do not settle, are mostly microscopic, and cannot filtered easily.
An emulsion is a mixture of two liquids.
Foam is formed when many gas particles are trapped in a liquid or solid.
Aerosol contains small particles of liquid or solid dispersed in a gas.
Suggested procedures overview :
- Put learners into groups or have learners take turns to demonstrate the investigation.
- Ask. What is a mixture?
- Ask. What is a solution?
- Explain, with a model how solutions or mixtures change according to the difference in size of the components that make up the solution. Changes it properties: looks, transparency, flow ...
Exploration to review / assess what learners know about properties of a mixture and solution.
- Put learners into groups or have learners take turns to demonstrate the investigation.
- Ask. What happens when water is added to sugar? it dissolves
- Ask. What other solids are similar to sugar? salt, tea, coffee
- Ask. What do you think will happen with these solids: soil, flour, cocoa, tea, coffee, pepper, baking soda, and baking powder? Accept all answers. No need to correct ... They will dissolve and make a solution
- Ask. If they think the temperature of the water would make a difference? yes, no, maybe
- List each compound on the board and have them record what they think will happen if they are mixed in hot or cold water.
- Demonstrate how they should put a small amount of soil (teaspoon) in two clear cups. And how much warm water and cold water (1 cup) to add to each. Don't add it just demo.
- Check for understanding for their procedure and how they are going to record the results. How not to stir for so long and when to stir and for how long.
- When everyone is ready have them repeat their procedure with each substance.
- Have them begin their procedures and record their results in their lab notes.
- When all are completed, tell them to let all the mixtures sit for five minutes. Record what (if anything) happens after it has been sitting.
- Ask. Them to compare their findings.
Invention
- Have them discuss why some compounds mixed differently.
- Then discuss what difference there was with warm water instead of cold water.
- Ask. Are all of these mixtures?
- Ask. How can you interpret the results?
Discovery
- Ask. Where could you use what you learned?
- Next ...
Activity 9 - Skittle chromatography
Materials
- Skittles, plate, water
- Lab note
Focus questions :
- What happens when a substance is place in water?

Learning outcomes
Describe how the skittle is a mixture, dissolves when a solvent (water) is added, and becomes a solution. Then describe how it acts as a solute and flows through the solvent (water). Then as the water evaporates changes back to a Skittle? or something else?
Suggested procedures overview :
- Put learners in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity - Set up the skittle experiment.
- Describe how the skittle is a mixture, dissolves when a solvent (water) is added, and becomes a solution. Then describe how it acts as a solute and flows through the solvent (water). Then as the water evaporates changes back to a Skittle? or something else?
Exploration
Activity:
- Put learners into groups.
- Ask. What happens when a substance is placed in water? Accept all answers. Depends on the substance. How it may change to a solution ...
- What about skittles? Accept all answers. Depends on the substance. How it may change to a solution ...
- Set up the experiment.
- Observe ... Not sure how long as haven't skittled this ...
- Observe and draw a diagram of what happens, melting solution, evaporate and back to a mixture.
Invention
- Share results.
- Describe how the skittle dissolves in the water and cohesion and adhesion cause the dissolved substance (solute) to flow and become a solution. Then the water evaporates and the solution becomes a solid mixture.
- Introduce chromatography ... is the separation of a mixture by passing it in solution (or suspension or gas) through a medium (filter paper) in which the components flow at different rates.
Add to or edit Properties of liquids ...
Discovery
More activities on chromatography like the shark activity ...
Can use coffee filters to make good good strips to place in different liquids or to draw on with a marker, insert into water, and let the good times flow!
Lab Notes for activities
Activity 1 - Paper clips & coins
Materials :
Focus questions :
How do we separate a group of mixed objects??
Challenge:
How can you separate the coins and paper clips?
Draw a picture to show how a different property is used to separate components in a mixture.
Activity 2 - Salt & sugar mixture
Materials
- Salt crystals, sugar crystals, cups, magnifying lenses
Focus question:
How can you identify salt and sugar?
Use a hand lens or microscope to draw a picture of each and label their properties:
Salt
Sugar
After you mix salt and sugar what did it taste like?
Why is it a mixture?
Activity 3 - Make a mixture or separate a mixture
Materials
- Food items provided.
Challenge
Use the selected food item or items and either make a mixture or separate a mixture into its components.
Before picture
After picture
Describe how the properties of the components changed or didn't change from a mixture or separate components of a mixture.
Activity 4 - Properties of solutions
Possible materials:
- Water, liquid hand soap (white), dish soap (blue or green), oil, Italian salad dressing, three clear bottles. Make three different solutions: oil & water, soap & water, Italian salad dressing, and juice (fruit or vegetable).
Focus question
What are the properties of solutions?
Explore and describe the properties of solution _____________________
Explore and describe the properties of solution _____________________
Explore and describe the properties of solution _____________________
Make a list of solution properties.
Activity 5 - Mixing liquids
Materials :
- Different liquids
Focus questions
What happens when different liquids are mixed?
Directions:
- Get two bottles with lids.
- Select two different liquids to put into each bottle.
- Put two different liquids into each bottle.
- Describe the liquids properties before.
- Describe the properties after being mixed, how the properties change or don't change, and how they interact.
Bottle 1
Before diagram and its properties
After diagram, its properties, and how the properties interact. (float, sink, mix, ...)
Bottle 2
Before diagram and its properties
After diagram, its properties, and how the properties interact. (float, sink, mix, ...)
Activity 6 - Sugar & water
Materials
Teaspoon, shallow plate or container, sugar, water, and a small container to mix them.
Focus questions
What happens when sugar and water are mixed?
Before:
Sugar
Water
After
Sugar water
Challenge
How can you separate them?
Procedure to separate.
Before
After
Activity 7 - Sand & salt water
Materials :
- Sand, tablespoon of salt, drinking glass, water, coffee filter, plate
Focus questions
What happens when sand, salt, and water are mixed?
Draw and explain ... use their properties ...
Before
After ....
After ....
Activity 9 - Skittle chromatography
Materials
- Skittles, plate, water
Focus question
What happens when a substance is put into water?
Challenge
Create the following ...

Explain!
Describe how the skittle is a mixture, dissolves when a solvent (water) is added, and becomes a solution. Then describe how it acts as a solute and flows through the solvent (water). Then as the water evaporates changes back to a Skittle? or something else?
Graph paper
Fact sheet - Properties of mixtures & solutions - fact sheet
- A mixture is a substance made by mixing other substances together and can be separated into the substances from which they are made. Mixtures and their substances can be combinations of solids, liquids and gases. Like carbonation in drinks, air between spaces of solid and liquid mixtures. The particles are generally larger than those in a solution. Substances can be separated using differences between one or more of the their properties (sifted, screened, filtration, evaporation, magnetism, electrical charge, ...).
- A solution is a liquid mixture where solid particles are said to dissolve in a liquid and become uniformly distributes. The substance that dissolves is called a solute. The liquid in which it dissolves is called a solvent. The particles are generally smaller than those in a mixture. Solutions can have particles (solute) of various sizes. However, solution is a term generally used when the particles of the solute can be seen with your naked eyes. Thus, there are different names for solutions with different sizes, and people may use these terms interchangeably (solution, suspension, colloid).
- Solutions are matter that take up space and have mass. It exerts a force and flows when not at equilibrium.
- The different substances in a solution can mix really well or separate when not being shaken.
- Some will stay mixed and others will separate at different rates.
- Solutions flow and spread out.
- Different kinds of solutions, depending on how thick they are, flow (spread out) at different rates,
- Solutions will flow until they interact with another object.
- When they interact with another object they can change their direction or be contained.
- Usually solutions are contained in a solid container.
- Solutions which are contained will take the shape of the container.
- When solutions are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- Solutions can be: transparent, translucent, viscous, opaque, foamy, and bubbly.
- Dissolve means to get smaller and become part of a solution. It is only a physical change of getting smaller, as opposed to a chemical change of becoming another substance. Particles can vary in size from being visible to microscopic to being single atoms or molecules.
- Solvent is a liquid that can dissolve a substance (the solute) to make a solution.
- Solute is a substance that a liquid can dissolve to make a solution.
- Mixtures and solutions are made of matter that takes up space and have mass. It exerts a force and flows when not at equilibrium.
- Solutions flow and take the shape of a container. And if they are not in a container, they will flow at different speeds (rates) and some will spread out more than others.
- Chromatography is the separation of a mixture by passing it in solution (or suspension or gas) through a medium in which the components flow at different rates.
- Different concentrations may have different or similar densities.
- Concentration is the amount of a given substance within a solution.
- A suspension is a solution of two or more substances where the solution is opaque to light, the particles are visible to the naked eye, and settle down under the influence of gravity. A suspension has particles generally smaller than those referred to as a solution and can be separated out by ordinary filtering or centrifuging.
- Colloids are suspensions of two or more substances where the solute particles remain suspended throughout the solution (bouillon cube, milk, paint, blood, fog, whipped cream, perfume, gels) and is often transparent or translucent to light. Some colloids can be classified on the basis of the state of matter (emulsion, foam, aerosol) of the dispersed substance and the state of matter in which it is dispersed. The colloidal suspension with solid particles in a liquid is known as colloidal solution. the particles do not settle, are mostly microscopic, and cannot filtered easily.
- An emulsion is a mixture of two liquids.
- Foam is formed when many gas particles are trapped in a liquid or solid.
- Aerosol contains small particles of liquid or solid dispersed in a gas.
Science directories
- Science investigations
- Concepts - knowledge base
- Pedagogy
- Main index