Pressure - Air & balloon explorations
A unit with lesson plans, activities, & lab notes
Rough draft ...
Introduction
- Introduction
- Big ideas, concepts, facts, & outcomes
- Science content concepts & outcomes
- Inquiry & science process concepts
- Pedagogical overview
- Activities sequence
- Focus questions
- Materials
- Scoring guide suggestions
- Unit activity sequence
- Activity 1 -
- Activity 2 -
- Activity 3 -
- Activity 4 -
- Activity 5 -
- Activity 6 -
- Lab notes
- Lab note 1 -
- Lab note 2 -
- Lab note 3 -
- Lab note 4 -
- Lab note 5 -
- Lab note 6 -
- Support materials
A sequence of plans to facilitate a review and develop a deeper understanding of .
Related information
Planning information
Learner background information
A plan designed for learners who have prior knowledge in cause and effect, use of observations to make inferences, models as explanations for observble and non observable events, relative postion, and working in groups.
Intended learnings & learners thinkings
Content Big ideas, concept & facts, & outcomes
(Source concepts & misconceptions)
Big ideas and specific outcomes:
A
Concepts and facts
Outcome
Use and describe
Anticipated learner thinkings & misconceptions
T
Scoring guides suggestions (rubric)
(scoring guide)
Top level
- A
Lower level
Inquiry, process, & cross cutting concepts & skills
Inquiry (How science inquires for understanding: process, skill, methodology, practice)
When I experiment I collect observations that describe how different properties change (become variables) when objects and systems interact. This helps me make claims to explain what is happening, and make models to predict what might happen in the future.
Related concepts and facts
- Observed changes can be described as changes of properties (variables).
- Variables are observations of a property that changes - size, shape, temperature, amount, volume, rate, ...
- When people disagree on an observation, they usually make more and better observations.
- Observation, creativity, and logical argument are used to explain how variable changes effect resulting observations.
- Observed changes can be explained as being caused by changes of variables (changes of properties/ characteristics).
- Explanations are based on observations.
- Evidence is observation.
- Inference is an explanation based on observation.
- When people disagree on explanations, they can make more observations or change their explanation.
Outcome
Describe change as a result of interactions. Describe those interactions as changes of a characteristic/ property (variable) that interacts with the object that changes.
Specific outcomes -
Cross cutting
Systems, Order, & Organization; Evidence, Models, and Explanations; Constancy, Change, and Measurement; Evolution and Equilibrium; Form and Function
Stability and change - when objects exert equal forces on each other they are at equilibrium. When objects are not in equilibrium one will displace the other.
Systems & system models - Drawings can be created to represent and explain scientific ideas.
See more proceess concepts & misconceptions
Related concepts and facts
- Observational data and reasoning is used to explain interactions. Evidence is something that
is observed and can be used to understand what is happening and make predictions
about future changes.
- Explanations are based on observation derived from experience or experimentation and are understandable.
- Pictures or symbols can represent objects.
- Models are structures that correspond to real objects, events, or classes of events that have explanatory and predictive power (physical objects, plans, mental constructs, mathematical equations, computer simulations...) that may or may not be observed with real objects, systems, and events.
- Model is an explanation based on observations, facts, laws, inferences, thought, and reasoning.
- Models represent systems or things used as an example to follow or imitate to provide an explanation.
- Models can be used to think about events or processes that happen very slow, fast, or on a too small or large scale to change easily or safely.
- Mathematical models can be displayed on computers and changed to see what happens.
- Models are used to represent things in all dimensions of science physical, earth, and life science.
- Models are created with similar processes, which can be an algorithm or procedure. Such as in the diagram.
- A model, though different from the real thing, can be used to learn something about the real thing.
- Seeing how a model changes may suggest how the real thing works if the same were done to it.
- Models make predictions.
- More than one model can represent the same thing or event.
- The kind of model and its complexity depend on the purpose of using the model.
- Models are never exact representations.
- A model that is too limited or complicated may not be useful.
- A model represents entities and the relationships between them.
- There are two basic types of models, physical and conceptual. Conceptual communicate through words and drawings, or can be physical and demonstrable.
- Models help generate ideas, solve problems, make predictions, help think.
- Models can be used to represent new ideas and inventions.
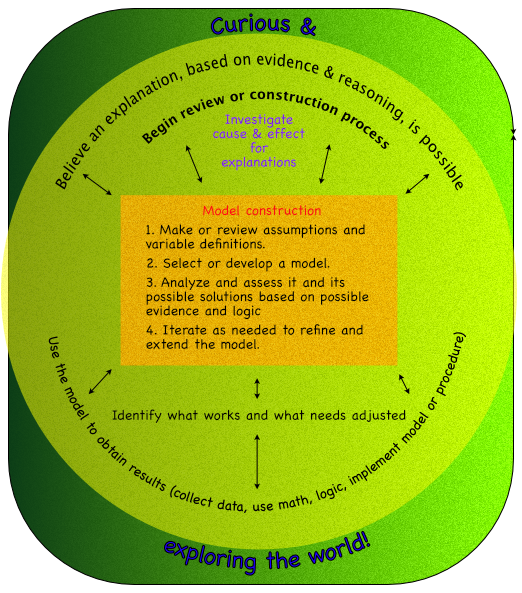
Outcome -
- Make observations of change, identify variables associated with the change, and create explanations of how the variables effect those changes.
Specific outcomes -
- Relate variables to change with an explanation.
Perspectives
Engineering & Technology
- People have always had problems and invented tools and techniques (ways of doing something) for scientific inquiry and technological design.
- The two have similarities and differences.
- Scientists propose explanations for questions about the natural world, and engineers propose solutions relating to human problems, needs, and aspirations.
- Technologies exist within nature and so they cannot contravene physical or biological principles. Technological solutions and technologies they have side effects, costs, carry risks, and provide benefits.
- Many different people in different cultures have made and continue to make contributions to science and technology.
- Technology is essential to science, because it provides instruments and techniques that enable observations of objects and phenomena that are other wise unobservable due to factors such as quantity, distance, location, size, and speed.
- Technology provides tools for investigations, inquiry, and analysis.
- Science and technology are reciprocal.
- Science helps drive technology, as it addresses questions that demand more sophisticated instruments and provides ideas for better instrumentation and technique. Technological designs have constraints that engineers, architects, and others must take into account to solve practical problems.
- Some constraints are unavoidable, for example, properties of materials, or effects of weather and friction; other constraints limit choices in the design, for example, environmental protection, human safety, and aesthetics.
- The knowledge base for science is recorded in print and electronic media and can be found and understood by people in and out of classrooms
Personal and Social Science
- The application and the use of models in science and technology can and will benefit society.
- Model helps me internalizing or abstracting understanding.
Nature of Science
- Science can provide ideas for our protection and desire for a better life.
- Scientists use models.
History of science
xSee also Concepts & misconceptions also science, math, technology timeline
- People have practiced science and technology for a long time.
- Science develops over time.
- Science investigators such as
Strategies and activities to achieve intended learnings
Based on learning cycle theory & method
Pedagogical Overview
Matter occupies space. No two objects can occupy the same space at the same time. Matter can be pushed by solid objects, liquids and gases that have a greater force. There is a lot of air above us pushing down. Solids, liquids, and gases exert more pressure at the bottom than at the top. Pressure inside a closed system with a fluid substance will exert a force in the fluid in all directions.
- Pressure is a force exereted over an area.
- Gases and liquids flow when they exert unequal pressure on each other.
Activities Sequence to provide sufficient opportunities for students to achieve the targeted outcomes.
Make sure learners have the prior knowledge identified in the background information.
- start with water in a plastic bag and pencil pierce activity
- Activity 1 - Intro
- Individually answer the focus questions in a science journal
- Blow up a balloon
- Draw a diagram to explain the pressures on the balloon
- Activity 2 - Demo - Blow up a ballon inside a two-liter plastic bottle; Draw a diagram to explain the pressures on the balloon and bottle.
- Activity 3 - Challenge - Blow up the balloon inside a two-liter plastic bottle with a straw; Draw a diagram to explain the pressures on the balloon and bottle
- Activity 4 - Put a tied balloon with air in it into a two-liter plastic pop bottle and use the fizz keeper® to add air to the bottle; Draw a before and after diagram to explain the pressure on the balloon and bottle
- Activity 5 - Look inside a balloon; Draw a diagram to explain the pressure on the balloon and bottle.
- Activity 6 - Water and air pressure?
- Water pressure and air with a lid ... see ...
- Water pressure and air without a lid ... see ...
- Activity 7 - Cartesian diver - video and discussion as discrepant event
Focus question
Unit focus question:
- What is pressure?
Sub focus questions:
- What are the properties of presssure?
- What variables affect pressure?
- What is equilibrium?
- Can pressures be at equilibrium?
- What happens if pressures aren't at equilibrium?
- How can pressure be represented in diagrams?
- How can systems, subsystems, and models be used in explanations?
- How can science be used to answer real world questions?
Materials
- Lab note 1 -
- Lab note 2 -
- Lab note 3 -
- Lab note 4 -
- Lab note 5 -
- Lab note 6 -
Resources
Lesson Plans
Activity 1 -
Materials
Focus questions:
- What is
Learning outcomes:
- Explo
Suggested procedures overview:
- Put learners in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity -
Exploration -
- Put learners in pairs.
- Ask.
Invention -
- Explain and discuss with the learners
Discover
Activity 1 Focus Questions
Materials:
Procedure:
- Ask students to answer the following questions individually in their science notebooks.What is pressure?
- What are the properties of presssure?
- What variables affect pressure?
- What is equilibrium?
- Can pressures be at equilibrium?
- What happens if pressures aren't at equilibrium?
- How can pressure be represented in diagrams?
- How can systems, subsystems, and models be used in explanations?
- How can science be used to answer real world questions?
Activity 2 Blow up a Balloon
Materials: Balloon
Procedure:
- Ask the students to blow up a balloon and explained what happened.
- Keep it untied because they will use it again.
Activity 3 Draw a diagram to explain the pressures on the balloon.
Let the air out of the ballooon. Explain what happened. Why did the air leave?
Activity 4 Blow up a ballon inside a two-liter plastic bottle
Materials: Balloon, two-liter bottle,
Procedure:
Activity 5
Draw a diagram to explain the pressures on the balloon and bottle.
Activity 6
Blow up the balloon inside a two-liter plastic bottle with a straw
Materials: :
Balloon, two-liter bottle, straw
Procedure:
1.
Activity 7
Draw a diagram to explain the pressures on the balloon and bottle
Activity 8
Put a tied balloon with air in it into a two-liter plastic pop bottle and use the fizz keeper® to add air to the bottle
Materials:
Balloon, two-liter bottle, fizz keeper®
Procedure:
1.
Activity 9
Draw a diagram to explain the pressure on the balloon and bottle
Activity 10
Look inside a balloon
Materials:
Balloon, two-liter bottle with two tops
Procedure:
1.
Activity 11
Draw a diagram to use to answer the following questions for activity 10:
Is there pressure?
What are the properties of presssure(s)?
What variables are effecting the pressure(s)?
Are the pressures at equilibrium?
How did using a diagram help you represented pressure?
Activity 12
Air pressure and change of volume
Materials: 2 liter plastic bottle, hot water, lid.
Procedure: Fill the bottle with hot water, dump the water, cap the bottle quickly and allow it to stand.
Activity 12
Air pressure and water
Materials: 2 liter bottle, push pins.
Procedure: Fill the bottle with water, cap the bottle, stick two pins into the bottle one about an inch below the water level and the other below the first. Remove one pin replace, remove the other, remove both, and then try with the cap unscrewed. What if the two holes were both two inches below the water level?
Activity 13
Pressure changes with the amount of matter
Materials 2 liter bottle, push pins, measuring device.
Procedure: Fill the bottle with water, make two - three pin holes at 2 cm intervals from the bottom of the bottle, put masking tape on each hole, remove one piece of tape from a hole and collect the water for a predetermined amount of time, refill the bottle and repeat for other holes.
Activity 14
Pressure changes the volume and density of matter.
Materials: Glass eye dropper, 2 liter plastic bottles with cap.
Activity: Make the eye dropper rise and fall in a bottle of water.
Activity 2 -
Materials:
Focus questions:
- How do we
Learning outcomes:
- Describe
Suggested procedures overview:
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity -
Exploration
Invention
- Recall and review
- What groups of
Discover
Activity 3 -

Materials:
Focus questions:
- What are
Learning outcomes:
- Make
Suggested procedures overview:
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity -
Exploration
- Put learners in pairs.
- Ask.
Invention
- What
Discovery
- Discuss how
Activity 4 -
Materials
- S
- Lab notes -
Focus questions:
- How does
Learning outcomes:
- Learners
Suggested procedures overview:
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity -
Exploration
- Ask.
Invention
- Ask. How
Discover
Activity 5 -
Materials:
- M
- Lab notes -
Focus questions:
- What
Learning outcomes:
- Explain
Suggested procedures overview:
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity -
Exploration
- Organize learners into pairs and groups.
- Have learners
Invention
- W
Discover
- If
Activity 6 -
Materials
- T
- Lab notes -
Focus questions:
- How do
Learning outcomes:
- Describe
Suggested procedures overview:
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity -
Exploration
- Organize learners into groups and pairs.
Invention
- Regroup as a class and groups demonstrate their projects.
- Ask.
Discover
Lab Notes for activities
Home: Pedagogy - theory, curriculum, learning, human development, & teaching
Home: Science - knowledge base, activities, pedagogical knowledge in all dimensions