Energy
Conservation & limits to energy, Combustion, & Fire triangle
Questioning is the foundation of all learning.
The first step in rejecting not knowing is to ask, why?
Sweetland
Overview
Introduction
This page includes a starter plan to facilitate a review and develop a deeper understanding of conservation, natural resources, and their functions to understand them and make better decisions when critically thinking about conservation.
Activities include challenge for heating water with a candle, combustion, and fire triangle.
Background information:
Energy resources can be grouped as nonrenewable, renewable, and synthesized.
The limit of a nonrenewable resource is the amount of a resource that can be found and used at a reasonable cost and marginal environmental impact. In short, it is the quantity of the resource that defines the limits to its use. By analogy using a nonrenewable resource is like living off your savings when you have no income. When the savings is gone, your income is gone.
Renewable resources are, in theory, limitless. But in fact, there is a limit. If a renewable resource is used faster than it can be replenished, then the resource becomes depleted and ultimately is nonrenewable. Again, by analogy, using renewable resources is like living off the interest of your savings without using the capital. If use exceeds the interest rate, then capital is diminished at your rate of expenditure until your capital is depleted. In the case of a renewable resources it is the rate of use that is the limiting factor.
Energy must be available in useful form, at reasonable costs, and without harmful effects on the environment. Energy scarcity, then, does not really mean we are running out of energy sources. It means that there may be a shortage of a resource, a rise in price (such as happens when OPEC increases the price of crude oil) or a substantial amount of environmental damage.
Societal implications
Oil and gasoline prices have risen since 1973. The oil embargo made individuals aware of another fact: the dependency of other social factors on petroleum. Energy is needed for much more than cars. Energy is needed to run machines and grow food; and, if the source of energy is oil and it becomes more expensive, so does all the products for which oil is a source of energy.
1973 was a turning point for industrialized countries. During the period from 1973 to 1980 we became aware of the limits to our energy resources. If we take the lesson of limits seriously and start the transition to conservation practices and alternative sources, all will be well. If, on the other hand, we do not face the reality of limited energy resources, our society will be in serious trouble.
Adapted from: Bybee, Peterson, Bowyer, and Butts. Science and society/activities. Charles E. Merrill Publishing Co. A Bell & Howell Company
Related study topics:
- Heat
- Heat and energy transfer - teacher directed plan with lab notes to explore: hot & cold with touch, variety of activities using water, mixing hot & cold water, warming ice water to boiling, salt & ice, temperatures of cars in sunshine, blowing hot & cold bubbles, conduction, convection, & radiation.
- Temperature & thermometers - A motivational challenge to explore how to measure temperature and decide on a degree of accuracy. Thermochromatic materials are introduced and used to increase interest and focus.
Pedagogical notes
Big ideas, concepts, facts, and outcomes
Big ideas
- We depend on energy transfer for light, heat, and other uses to be available for our daily lives. However, to sustain a supply of energy that is available to all at reasonable costs we must know how to manage and conserve our resources.
Related concepts and facts
- Better decisions are made when information is verified before being considered accurate and used to reason and develop explanations and models to understand the world and make decisions.
- People make better decision when they understand and consider the positive and negative influences that effect their decision making.
Outcome
Use accurate verifiable information to make energy decisions.
Science physical, earth, life
Big ideas: Sources of energy are limited.
Related concepts
- Some sources can only be used once (nonrenewable).
- Renewable resources can be replenished.
- Conservation is one method of using less energy.
- Conservation is using less.
- Conservation is the preservation of resources through decreased use.
- Changing energy sources is one method of reducing the use of certain kinds of energy.
- Some energy sources are more abundant than others.
- Depletion of resources can only be stopped by
- decreased use and
- increased supply.
Outcome
- Explain how resources are limited and how to conserve their use
Science inquiry, process, & perspective concepts, facts, & outcomes
Big ideas: Energy transfer causes a change, but those changes have some constancy.
Transfer of energy will occur until there is equilibrium.
Related concepts and facts
- Observational data can be used to explain ideas and answer questions.
- Technology can be created using scientific ideas.
Concepts, misconceptions, & outcomes, knowledge bases
Outcome
- Use observations to explain the transfer of energy in a burning candle system.
Activities Sequence to provide sufficient opportunities for students to achieve the targeted outcomes.
Make sure students have the prior knowledge identified in the background information.
- Activity 1 - Students Brainstorm from their prior knowledge about the unit focus question, and discuss sub focus questions as appropriate to set unit learning goals.
- Activity 2 - Candle system energy transfer: chemical, light, heat ... Challenge
- Activity 3 - Fire triangle
Focus question
Unit focus question:
- What is energy?
- Can energy be reused?
- How do we get energy?
- Can energy be reduced?
- What kinds of energy use can be reduced?
- What is need for a fire?
Sub focus questions:
- What kinds of resources are there?
- What kinds of energy are involved in burning a candle?
- How is energy transfered?
Resources and Materials
- Lab note 1
- Lab note 2 -
- Lab note 3 - Fire triangle
Fact sheet
Candle illustration with explanation of energy transfer in the candle system.
Scoring guides suggestions (rubric)
Energy conservation(scoring guide)
Top level
- Explains how energy is transfered in an energy system and how all the variables or properties necessary for the system to operate need to be conserved to reduce their use.
- Focuses on one variable and explains how it is necessary to change to conserve energy.
- Manipulates one variable without explanation as why it is selected.
Lower level
Lesson Plans
Activity 1 - Focus activity
Materials:
- Brainstorming guidelines, page one in lab notes or blank sheet of paper,
- Small birthday candles, matches, Styrofoam cups, clock, thermometer, test tubes, and test tube holder for each group of learners.
- Appropriate safety materials: Goggles, fire extinguisher, or container of water …
Focus questions:
- How can you transfer the most energy from a candle to water?
- How do p
Learning outcomes:
- Explain how heat energy is transferred and how to achieve efficient transfers.
Suggested procedures overview:
- Demonstrate heating water with a candle.
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity challenge learners to devise a procedure to conserve energy to heat water with a candle.
Exploration -
- Before class place a small hole in the bottom of the Styrofoam cup and place a small birthday candle in the bottom of the cup to make a candle holder.
- Tell the learners you are going to use the candle to heat water in a test tube.
- Put 5 cm (10 ml) of water at room temperature in the test tube.
- Ask. How long do you think it will take to heat the water and increase the temperature by 10 degrees Celsius?
- Ask. How much of the candle will be used?
- During the activity demonstrate how to record:
- The time it takes to heat the water 10 degrees C.
- How much (in centimeters) of the candle is used. Or mass in grams.
- Discuss results.
- Ask. How long do you think it would take to heat the water 20 degrees C? 30 degrees C?
- How much of the candle would be used for each?
- How long would the candle last?
Invention - Challenge
- Challenge the learners to ask how the the water could be heated 30 degrees C using the least amount of candle.
- Brainstorm different ways to do it.
- Select procedures to test.
- Test.
- Share and discuss results.
- During the discussion list their ideas on a screen or board and classify them according to those which represent conservation of the energy source and those that represent use of other energy sources and combinations of the conservation and use of renewable resources.
- Extend the discussion to the limits and conservation of natural resources such as fossil fuels.
- Have the learners design their own activity to demonstrate more efficient methods to use energy.
Activity 2 - Relight a candle Energy as chemical, heat, light, ...
Materials:
- Candles, ignition source
- Appropriate safety materials: Goggles, fire extinguisher, or container of water …
Focus questions:
- What makes the energy? Light, heat, chemical, ... ?
Learning outcomes:
- Learners will suggest sources of energy and how energy is transfered from one type to another.
Exploration - Demonstration
- Tell learners you are going to demonstrate how to relight a candle and want them to consider what is happening in the system as energy is transfered.
- DEMONSTRATION with Relight a candle.
- Burn a candle long enough so the wax is melting.
- Light a second candle.
- Blow out the first candle and hold both horizontal with the lite candle flame a few centimeters below the wick of the recently extinguished candle.
- Within a few seconds the candle should relight.
Invention
Hint: Solid candle wax melts, vaporizes and the vapor rises and is burned by the flame at the wick. Normally when a candle is lit, the wick burns until it is hot enough to melt and vaporize the wax, then the wax vapor is burnt. The flame provides enough heat to continually provide fuel for the flame. Source
Could use a cold third candle and demonstrate with it the same way to show or see that it won't light or if it does, it takes a significant longer time.
Discovery
Next activity - fire triangle and combustion
Activity 3 - Fire triangle
Materials:
- Lab notes 3
Resources
Focus questions:
- What is needed for a fire?
Learning outcomes:
- Learners will describe that a fire needs heat, fuel, and oxygen to burn and describe how removing one of the three will extinquish a fire.
Exploration - Demonstration
- Ask. what was need for the candle to relight? heat
- What is needed for a fire? accept all answers
- Display a fire triangle.
- Ask. How are the three elements necessary for a fire? fuel heats up until it compusts meaning it oxidizes with oxygen and changes chemically
- Continue discussion and complete lab note 3 using resources as they fit with the discussion.
Lab Notes for activities
Activity 1 - Brainstorming & goal setting
- Accept all suggestions (no criticism).
- Free wheeling or hitch-hiking is allowed and encouraged.
- Generate a large number of ideas.
- Combinations and improvements are sought.
- Everyone says their idea out loud and each writes their own ideas.
- The wilder the idea the better.
Materials
- Draw a diagram of the candle system.
Explain the source, receiver, and energy transfer.
Describe how the amount of candle can be conserved and the amount of heat energy transferred to the water can increase the temperature the same.
Challenge
Increase the temperature of 10 ml of water to the highest temperature in two minutes.
Activity 3 - Fire triangle
Resources
Challenge
- Draw a diagram of a fire pit and identify the three elements (main variables) to keep a fire burning.
The three variables are said to be the fire triangle and to extinquish a fire, you need to remove one of the three.
Consider these examples and describe how they affect combustion..
Putting another log in the fire pit.
Blowing out a birthday candle.
Why a fire pit is in a metal container or has rocks around it.
Putting a lid on a pan of an oil fire.
Putting water on a fire.
Using kindling to start logs on fire.
Summary
Fires can be extinguished by
Explain how combustion fits with the ideas of a source, receiver, and energy transfer.
Resources
Fire triangle
Identify the elements necessary for a fire.
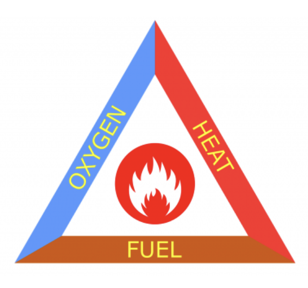
Compustion and extinguishiing fires
Combustion is a chemical reaction that feeds a fire more heat and allows it to continue burning or oxidizing. Once a fire has started, the resulting exothermic chain reaction allows it to continue, until at least one of the elements of the fire is blocked.
Ways to feed combustion
- Putting another log in the fire pit.
- Blowing out a birthday candle.
- Why a fire pit is in a metal container or has rocks around it.
- Putting a lid on a pan of an oil fire.
- Putting water on a fire.
- Using kindling to start logs on fire.
Extinguishinng fires
Foam- it reduces the oxygen.
Water - lowers the temperature of the fuel.
Remove or disperse the fuel. - removes the fuel.
Carbon dioxide can displace oxygen from the fire, because it is denser than air.
Halon can be used to remove free radicals and create a barrier of inert gas in a direct attack on the chemical reaction responsible for a fire.
What's a flame?

A flame is more than meets the eye.
In fact the part of the flame that enters your eye is light.
The part of the flame that you feel is heat.
The candle is solid.
The fuel, which is buring is a vapor.
If you look real close at the candle the bright spot of the flame you will see solid wax turning to liquid wax and liquid wax turning to gas wax (vapor). These changes happen differently depending on how close the wax is to the blue hot spot of the flame.
As the wax is heated, it changes to a gas and streams or flows with the air that travels up from the top of the candle into the flame stream where it becomes the flame and continues rising and exits the top of the system.
What it is in the flame, or becomes the flame, the wax gas mixes with air and changes from candle gas and oxygen in the air to carbon dioxide or some other oxide, some water vapor, and some left over candle soot or scent.
So a candle flame or other flame is the place in a stream of air where all the chemical, heat, and light action is really happening.
Inspired as an answer to Alan Alda's Challenge.