Metric measurement review
Matter activities & work sheets
Questioning is the foundation of all learning.
The first step in rejecting not knowing is to ask, why?
Sweetland
Introduction
This page includes plans for learner's to review and facilitate their understanding of measurement: linear, volume, mass, and density and their relationships to matter. It includes metric fact sheets, metric unit number line, model of a human hair and dust particle, and metric prefixes with exponential powers from +30 to -30.
Metric fact sheets
Metric units
Meter is the unit for linear measurement.
- One meter is a little longer than one yard.
- One millimeter = .001 meter or one thousandth meter (diameter of a paper clip)
- One centimenter = .01 meter or one hundredth meter (width of a paper clip or little finger)
- One kilometer = 1000 meters (a bit more than half a mile)
- 1000 mm = 1 m
- 100 cm = 1 m
- 1000 m = 1 km
Liter is the unit for volume. One liter is a little more than a quart.
- milliliter is .001 liters or one thoundth liter: Five ml is about a teaspoon.
Gram is the unit for mass. One gram is about the weight of a paper clip.
- kilogram is 1000 grams. One kilogram is about the same as a can of peaches about 2.2 pounds.
Most used prefixes for metric units:
- milli = one thousandth (.001)(10 -3)
- centi = one hundredth (.01) (10-2)
- kilo = one thousand times (1000)(103)
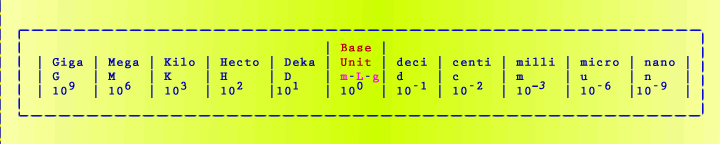
- More metric prefixes with exponents
Metric prefixes on a number line:
Model for a hair and dust particle size comparison

Metric prefixes, symbols, & exponential powers
| Prefix | Symbol | Power |
|---|---|---|
| Quecca | Q | 1030 |
| Ronna | R | 1027 |
| Yotta | Y | 1024 |
| Zetta | Z | 1021 |
| Exa | E | 1018 |
| Peta | P | 1015 |
| Tera | T | 1012 |
| Giga | G | 109 |
| Mega | M | 106 |
| kilo | k | 103 |
| milli | m | 10-3 |
| micro | μ | 10-6 |
| nano | n | 10-9 |
| pico | p | 10-12 |
| femto | f | 10-15 |
| atto | a | 10-18 |
| zepto | z | 10-21 |
| yocto | y | 10-24 |
| ronto | r | 10-27 |
| quecto | q | 10-30 |
See also: History of measurement
Activities
Linear measurement
Concept: Linear measurement is the measurement of lines (one dimension).
Background information:
- When using a ruler you count the SPACES between the lines.
- Prefixes:
- milli- thousandths,
- centi- hundredths,
- deci- tenths,
- kilo- thousand
Activities:
- Identify objects which measure approximately : 1 meter, 1 millimeter, 1 centimeter, 1 kilometer (Cognitive, comprehension).
- Identify the meter as the standard linear unit of measurement in the metric system (Cognitive, knowledge).
- Write the values of milli, centi, and kilo (Cognitive, knowledge).
- Measure objects in millimeters, centimeters, and kilometers (Cognitive, comprehension).
Volume
Concept: Volume is the measurement of the amount of space an object occupies. It may be asured in liters and cubic measurement
Background information:
- Liquids form a meniscus in cylindrical containers.
- There are two types: concave and convex.
- Read the scale from the top of a convex meniscus and from the bottom of a concave meniscus.
Activities:
- Identify objects with the volume of one milliliter and one liter (Cognitive, comprehension).
- Identify the liter as the standard unit for volume measurement in the metric system (Cognitive, knowledge).
- Find the volume of objects in milliliters and liters (Cognitive, comprehension).
- Find the volume of objects in cubic centimeters (Cognitive, comprehension).
Mass
Concept: Mass is the measurement of the amount of matter in an object.
Background information:
- Mass is measured with an equal arm balance.
- Weight is measured with a spring scale.
- Mass is not the measurement of gravity.
- Mass measures the amount of matter occupying a certain space (object in many instances).
- The gram and the kilogram are metric measures of mass and coincidentally only on Earth, weight.
- Sample problem: If an object on Earth weighs 600 grams its mass would also be 600 grams. If take this object to the moon, along with a spring scale and equal arm balance. It would weigh 100 grams, with the spring scale and 600 grams on the equal arm balance.
Activities:
- Identify objects with the mass of one gram and one kilogram (Cognitive, comprehension).
- Identify the gram as the standard unit for measurement of mass in the metric system (Cognitive, knowledge).
- Find the mass of objects in grams and kilograms (Cognitive, comprehension).
Density
Concept: Density is the relationship of mass to volume.
Density unit with plans & activities
Background information:
- Mass of liquids can be found by directly putting the liquid onto an equal-arm balance.
- It may also be found by finding the mass of a container, finding the mass of the container and liquid, and subtracting to find the difference.
- Volume can be found by submerging the solid in a know volume of water, noticing the increase in the water’s volume and subtracting to find the difference in volume, the volume of the solid object.
- Liquid volumes can be measured in a graduated cylinder. Caution students about dropping objects into graduated cylinders. They can easily be broken and some objects will get stuck. Also be careful to rinse the containers with soapy water when finished, as some liquids will discolor plastic containers as well as glass (alcohol and plastic).
- Some solid objects, for which to find density, are different metal objects like aluminum, lead, copper, and brass, different kinds of rocks, plastic, and glass. Some liquids to find density are water, salt water, glycerin, cooking oil, and alcohol.
Activities:
- Identify objects density as greater than, equal to, or less than one. (Cognitive, comprehension).
- Identify grams per milliliter as a unit for measurement of density in the metric system (Cognitive, knowledge).
- Find the mass of objects in grams and volume in milligrams (Cognitive, comprehension).
- Measure the mass and volume of different volumes of water and calculate its density (Cognitive synthesis).
- Measure the mass and volume of solid objects and calculate their densities (Cognitive synthesis).
- Measure the mass and volume of different liquids and calculate their densities (Cognitive synthesis).
Lab Notes for activities and Data Sheets
Metric Measurement and Matter Review Activities
Activity 1 - Linear Measurement
- Linear measurement is the distance between two points. This distance is a line and is in one dimensional.
- When using a ruler you count the SPACES between the lines.
What is the basic linear unit of metric measure?
What do each of the prefixes mean?
- milli
- centi
- kilo
Fill in the chart.
| 1 meter = | _____ millimeters |
| 1 meter = | _____ centimeters |
| 1 kilometer = | _____ kilometer |
Give an example of something that measures:
| Measurement | Item that measures ___. |
|---|---|
| 1 meter | |
| 1 millimeter | |
| 1 centimeter | |
| 1 kilometer |
Complete the chart.
| Object Name | Measurement in mm | Measurement in cm | Measurement in m |
|---|---|---|---|
| Width of paper | |||
| Length of paper | |||
| Width of desk | |||
| Length of desk | |||
The meter is used for what type of measurement?
Activity 2 - Volume Liquid Measurement
- Volume is the measure of the amount of space an object occupies.
- In the metric system the basic unit of volume is the liter.
- Liquid medicines are measured in milliliters.
Pour some water in a graduated cylinder.
Draw what you observe when you look through the cylinder at the top of the water.
You should have observed a meniscus.
There are two types: concave and convex.
Read the scale from the top of a convex meniscus and from the bottom of a concave meniscus.
Label your drawing from where you should measure.
Activity 3 - Volume Solid objects
Measure the volume of three rectangular containers.
container 1
container 2
container 3
What is the best metric measure for large bottles of pop?
What is the best metric measure for medicine?
The volume of one small white cube is one cubic cm or one ml.
Place three cubes together what is the volume?
What is the volume of a red cube?
What is the volume of a yellow cube?
What is the volume of an orange cube?
Another way to measure volume is to use linear measurements and calculate the volume with the linear measurement.
How is the volume of the above cubes calculated?
What is the measure for the amount of space an object takes up?
What are two ways of measuring volume in the metric system?
Activity 4 - Mass
The gram and the kilogram are metric measures of mass (weight on Earth).
What force are you measuring when you measure weight?
- Mass is not the measurement of this force, it is the amount of matter occupying a certain space.
- Mass may be measured using an equal arm balance.
- Weight may be measured using a spring scale.
What is the difference between these two methods?
Use a balance and record the mass of at least three objects.
| Name | Mass | |
|---|---|---|
| object 1 | ||
| object 2 | ||
| object 3 | ||
| object 4 |
Find and record an object with 1g mass. What object would have a 1kg mass?
BRAIN BUSTER: Imagine an object on Earth weighing 600 grams that would also be its mass. Suppose you launch this object to the moon, along with a spring scale and equal arm balance. What readings would you predict?
Spring scale
Equal arm balance
Putting Mass and volume to work
Use one of two methods to measure the mass of 50 ml and 100 ml of water.
Method one: indirect method
| Volume of Water | Mass of container | Mass of both | Mass of water |
|---|---|---|---|
| 50 ml | |||
| 100 ml |
Method two: direct method
| Volume of Water | Mass of water |
|---|---|
| 50 ml | |
| 100 ml |
Do the numbers need labels?
What pattern did you discover?
Use your data and calculate the mass for one ml of water?
Get an object (cube) and find its volume in cubic centimeters two ways.
Method one
| Describe how you found the volume. | The volume (did you label it?) |
|---|---|
|
|
Method two
| Describe how you found the volume. | The volume (did you label it?) |
|---|---|
|
|
All of these objects have what two properties?
Activity 5 - Mass & Volume
You have been measuring two properties of matter.
Look back at your data and put the information below.
| Mass of 50 ml of water | Volume of 50 ml of water |
|---|---|
How do the values compare?
The mass to volume ratio is called density.
mass / volume = density
Calculate a value with your data.
Look back at your data and put the information below.
| Mass of 100 ml of water | Volume of 100 ml of water |
|---|---|
How do the values compare?
The mass to volume ratio is called density.
mass / volume = density
Calculate a value with your data.
How do the values of the densities compare?
What is their mean?
The Class mean?
What does this value suggest about one ml of water?
Use cotton balls and a box to show density of 1 mass/unit
5 mass/unit
10 mass/unit, and
1.5 mass/unit.
Activity 6 - Density hunt
Question: What is the density of different matter?
Equipment: Balance beam, graduated cylinder, several different objects made of different matter, and a good eye.
Procedure: Get different objects and measure their mass and volume. Use this to calculate their density.
Results:
Date table
| Object | Mass | Volume | Density | Group Mean |
|---|---|---|---|---|
| Glass object | ||||
| Wood object | ||||
| Aluminum object | ||||
| 100 ml salt water | ||||
| 100 ml alcohol | ||||
| 100 ml of oil | ||||
Conclusions:
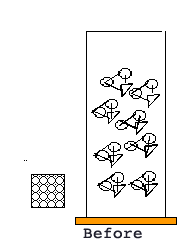
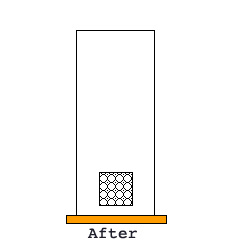
BRAIN BUSTER: If you measure mass and think of it as made from particles (cotton balls). Each cotton ball has a mass of one unit. If you have a box, with a volume of one, and it has one cotton ball in it, then it has a mass of one and volume of one and a density of one.
What would the density be of the same box with five cotton balls?
Using this analogy draw a picture of a box and label its mass, volume, and density.
Activity 7 - Density at work
- Steel has a density of 8g/cc and some metals that
have magnesium mixed in it (alloys) have a density of 2.5 g/cc. If you
build a race car why would you buy wheels built out of magnesium (mag)
wheels?
- I conducted an experiment the other day with an
egg. I put the egg in tap water and it sunk to the bottom. Next I put
the egg into some salt water and it floated. What would be the range of
densities for the egg?
- Model airplanes are made of balsa wood. How does
the density of balsa wood compare to other woods?
- A device called a hydrometer is used to test car
batteries to test the electrical charge. This device has a tube with a
round rubber ball on top and a weighted float inside. When you squeeze
the rubber ball, place it into a liquid and release the ball. Liquid will
be sucked into the tube. The float, which has markings on it, will float
in the liquid according to its density. If the battery is full of electricity
it will float at a level to show that the density of the liquid is 1.28
g/ml. If the battery doesn’t have enough electricity it will have
a density below 1.28 g/ml. If the battery is low on electricity will the
float higher or lower than when the battery is fully charged?
- Why do people who fish use lead weights?
- Suppose you have equal volumes of aluminum and wood. Which has more matter packed into it?
BRAIN BUSTER: Archimedes lived in the 3rd century BC, when according to legend, he was consulted by Hiero, King of Syracuse, to find out if his crown was solid gold. The story goes that the king hired a goldsmith to make a crown of solid gold, but either the king wasn't very trusting or the goldsmith did something to cause the king to distrust him. Any way he called his resident genius, Archimedes, and asked him to prove if the crown was solid gold or if the smith had cheated him by mixing silver with the gold when he made it. Archimedes must of thought long and hard and didn't come up with an answer until he was getting into his bath. Ah ha, light bulb lights up, and it is told that Archimedes ran through the streets of Syracuse shouting Eureka I have found it. Explain what Archimedes found?
Activity 8 - Review
Mass is measured in ________________
Volume may be measured in ______________ or
__________________ is the amount of space an object occupies.
__________________ is the amount of matter in a substance.
Everything in the Universe is made up of _______________
We used an analogy to imagine matter as small particles (cotton balls) a container as volume, and the relationship of the two as density.
People have wondered about matter for thousands of years. In the last few hundred years scientists have agreed on a theory of matter being made up of small particles called atoms or molecules. All matter is made of these small particles which interact as solids, liquids, and gases.
For example think of what happens to water (small particles held together weakly) when an aluminum block (small particles held together strongly) is placed into it.
First, think about the difference between a solid and a liquid? You know that liquids aren't held together as well as solids. When you pour water onto a table it flows into a puddle of water. If you put a block of ice on the table it will not flow. Imagine the difference between solids and liquids as the bond of energy between the particles. The picture below shows this. Each line represents one unit of force that holds the particles together. The more lines the harder they hold together.
![]()
Now back to the question: What happens when the aluminum block is put in water? Use the pictures to explain.
Metric Review
Answer the questions:
- Metric unit for length.
- Metric unit for mass.
- Metric unit for volume.
- Symbol for centimeter.
- Symbol for meter.
- Symbol for liter.
- Symbol for millimeter.
- Metric system is based on this number.
- 1000 milliliters is one.
- Scale used to measure temperature based on the temperature of freezing and boiling water.
- Water freezes at the celsius temperature.
- Liter is a unit to measure.
- Meter is a unit to measure.
- Gram is a unit to measure.
- You measure mass with this piece of equipment.
- A meterstick is about at long as a basketball players.
- This width of a paperclip measures one.
- Unit to measure distance.
- Symbol for kilometer.
- Pop is measured in.
- 1 meter is 100.
- 1000 millimeters is one.
- Liquid medicine for babies is measure in.
- Olympic weights are measured in.
- A meter stick measures.
- A measuring cup measures.
- One cubic centimeter of water has this mass.
- Measurement based on ten is known at this.
- Candy that is about one centimeter diameter.
Word bank
May use words more than once and their plurals:
- m&m
- meter, liter, gram, centimeter, milliliters, kilograms
- cm, ml, km
- one hundred
- celsius
- zero, one ten, hundred
- volume, mass, length, density
- distance
- balance
- arm
- metric system
Crossword puzzle
